32+ enthalpy of reaction calculator
Δ H r x n m H f p r o d u c t s n H f r e a c t a n t s. Web The first step is to find out how many moles of hydrogen peroxide that we have.
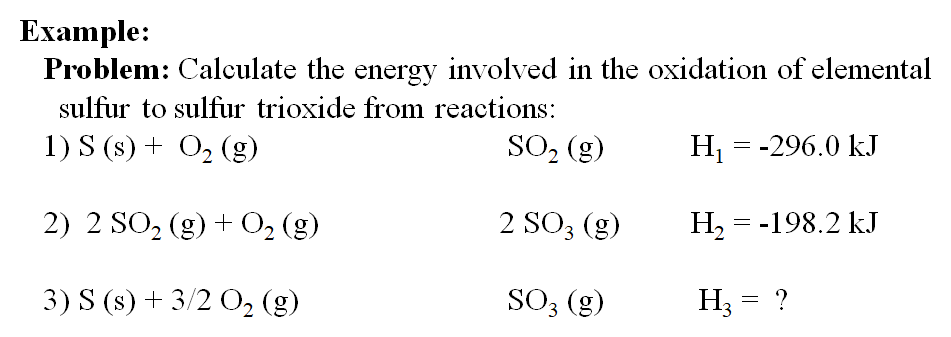
Chm1045 Enthalpy Lecture
Web Use the bond energies in the table to calculate the energy change for this reaction.

. 1 Show by calculation which reactant was in excess. So Hesss Law tells us that delta H of this reaction the change in enthalpy of this reaction is essentially going to be the sum of what it takes to. Web Free Chemical Reactions calculator - Calculate chemical reactions step-by-step.
H Q pV Where Q is the internal energy p is the vpressure V is the volume H is the enthalpy. Where Q internal energy. Web Enthalpy of combustion ΔH comb The change in enthalpy that occurs during a combustion reaction.
Web Calculate the standard heat of reaction Δ H o for the reaction of nitrogen monoxide gas with oxygen to form nitrogen dioxide gas. Enthalpy changes have been measured for the. Web Energy changes occur in chemical reactions as bonds are broken and new bonds formed.
Web Enthalpy Calculator Enter the required parameters and the calculator will calculate the amount of enthalpy generated in the heating system with the steps shown. Energy in 2. The formula to find change in enthalpy is ΔH ΔQ p ΔV.
So we take the mass of hydrogen peroxide which is five grams and we divide that by the molar. Web By knowing the reaction enthalpy for constituent reactions the enthalpy of a reaction that can be expressed as the sum of the constituent reactions can be calculated. Hesss law states that the net enthalpy of an overall reaction is.
Web Thats their heats of formation. Web 0 3. Web The formula to calculate the enthalpy is along the lines.
ΔH ΔH products ΔH reactants where ΔH is the standard enthalpy in Joules and ΔH products and ΔH. The enthalpy is calculated using the following formula H Q pV. Web The formula used to calculate the standard enthalpy is.
Web When we calculate If ΔHrxn we use this formula. Web The formula to calculate enthalpy is H Q pV. Web To find enthalpy using equilibrium constants you need to measure the equilibrium concentrations of products and reactants at two different temperatures.
Enthalpy changes can be calculated from experimental data and are independent of the route taken Hesss Law. Web Energy changes occur in chemical reactions as bonds are broken and new bonds formed. Use the data to calculate the experimental value for enthalpy of reaction in kJ mol.
Youll need starting and end values with constant pressure to calculate the change in enthalpy. 1Assume that the specific heat capacity of the solution is 418 J K1g1and the density. What is enthalpy of chemical reaction.
The enthalpy change formula is. ΔH Q₂ - Q₁ p V₂ - V₁ or ΔH ΔQ p ΔV. Enthalpy changes can be calculated from experimental data and are independent of the.
The key lies in the canceling of reactants and products that Ccur in the data reactions but not in the target reaction.

Enthalpies Of Formation Chemsitry Tutorial Youtube

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow
Enthalpy Changes

Monomeric And Trimeric Thorium Chlorides Isolated From Acidic Aqueous Solution Inorganic Chemistry
Enthalpy Calculator Calculator Academy

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow

Adding Reactions To Find Enthalpy Changes Youtube
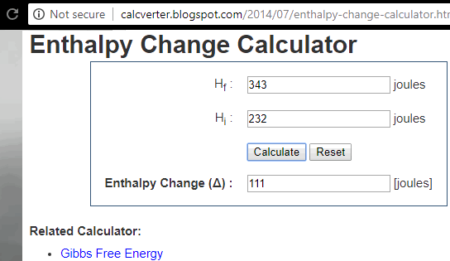
4 Free Online Reaction Enthalpy Calculator Websites

Enthalpy Calculator Calculator Academy

Monomeric And Trimeric Thorium Chlorides Isolated From Acidic Aqueous Solution Inorganic Chemistry
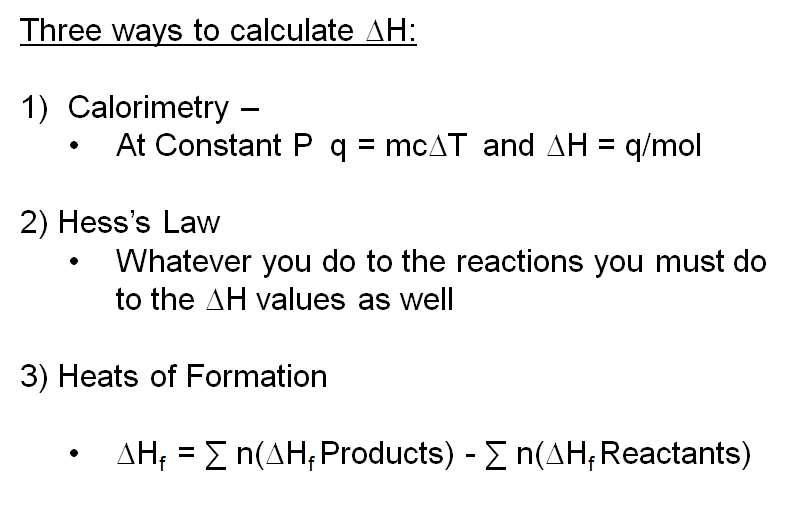
Chm1045 Enthalpy Lecture

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow

Doublet Ground State In A Vanadium Ii Complex Redox And Coordinative Noninnocence Of Tripodal Ligand Architecture Inorganic Chemistry

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow
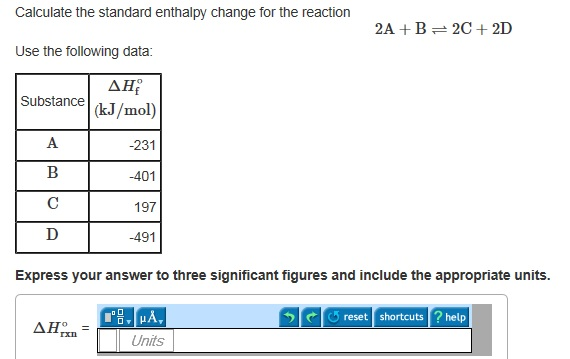
Solved Calculate The Standard Enthalpy Change For The Chegg Com

3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow